Chemical Effects of Electric Current NCERT Summary Class VIII Science Chapter 14
Good Conductor
What happens when electricity is passed through copper sulphate solution
The substance through which electricity can pass is called good conductor.
Examples - Copper, Aluminum
Poor conductor
The substance through which electricity can not pass is called poor conductor.
Examples- Rubber, plastic etc.
Tester
A device that test conductance of a substance, is called tester.
Solutions of acids, bases and salts are generally good conductor.
Chemical effects of Current
Chemical reactions (effects) caused when electricity passes through a conducting liquid, is chemical effects of current.
How to recognize chemical reaction
- Release of gas
- Change of colour of solution
- Release of heat
- Formation of new substance
In battery
No. of terminals- 2(positive and negative)
Positive terminal is also called Anode.
Negative terminal is also called cathode.
Solution is called electrolyte.
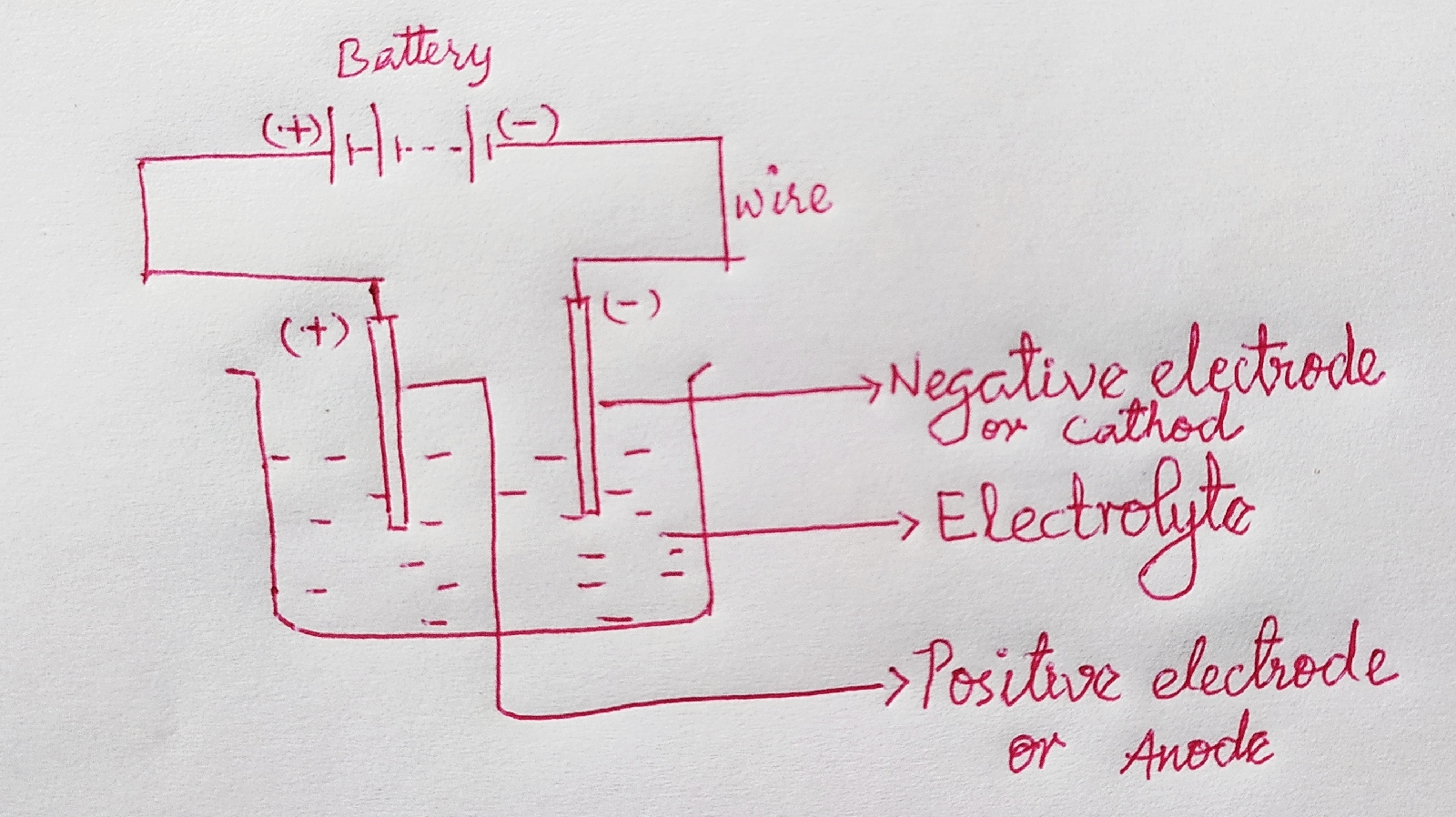 |
| Passing current through a solution |
Electroplating
The process of depositing a layer of any desired metal on another material by means of electricity, is called electroplating.
Plating by means of electricity.
Importance of electroplating
- Chromium plating on car parts, bath taps, kitchen gas etc
- Silver and gold plating on less expensive metals to make jewellery.
- Tin plating on iron to make tin cans.
- Zinc plating on iron used in bridges and automobiles.
Chromium plating is done because
- Chromium has shiny appearance.
- Chromium doesn't corrode.
- However, chromium is expensive. It may not be economical to make the whole object out of chromium.
Tin plating on iron is done because
- It doesn't corrode.
- Food doesn't come in contact with iron. Food doesn't get spoilt.
Zinc is coated over iron because
- Zinc protect iron from corrosion.
- Iron article doesn't get damaged.


Comments
Post a Comment